This is a student paper from the 2009 final projects in the NIH Foundation for Advanced Education in the Sciences’ TECH 366 — Biotechnology Management. The students were asked to tell a story based on the course lectures, and to expand with general lessons on biotechnology company management.
The changing roles of CRO
Contract research organizations (CRO) have played significant roles in the research and development (R&D) of biotechnology industry ever since its first appearance in the late 1970s. CRO provide important services in clinical trial management, safety monitoring, formulating and manufacturing, laboratory services, data management, NDA writing and filing as well as other regulatory affaire support, etc. The CRO industry had shown stable and increasing revenue on the global scale in recent years despite the weak growth of global economy. For instances, CRO shared $15 billions of global biotechnology R&D market in 2007 and $18 billions in 2008. In the United States, approximately $9 billion, accountable for 15% of total of R&D funds flowed to CRO in 2007. Even under the impact of global financial crisis, CRO revenue increased to approximately $10 billion when the total U.S. R&D increased to $65 billion in 2008 (Fig 1).
In recent years, the biotech industry has generated several lines of biomedical products that are sold over billion dollars each year, giving more room for CRO industry to grow. In 2008, for instances, monoclonal antibodies sales reached $33 billion, TNT blockers reached $18 billion, and erythropoietin reached $9.5 billions. The global sales of biotech drugs jumped from $75 billion in 2007 to $125 billion, almost 16% of global drug sales, in 2008. This fast growth (Fig 1) of biotech revenue may encourage R&D expenditure in biotechnology and therefore expand the market size for CRO in the near future.
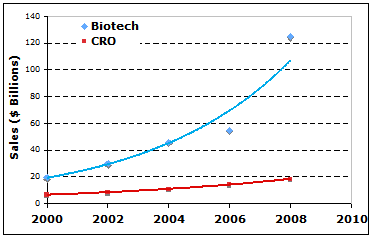
Fig 1. Global sales by CRO and Biotech industries. (data source: reference 1, 2, 3, 4.).
Today’s CROs may operate either as local niche services or on international scales. Several CROs, such as Quintiles translational corporation, Covance Incorporated, Pharmaceutical product development, Charles river labs and MDS incorporated, have generated over billion dollar revenues each in 2008. Out sourcing to CRO has become an attractive strategy for many biotech firms because CRO may provide services that are not available in-house for start-up firms. In some other cases, CRO may provide services with more competitive quality and better-targeted milestones than in-house research department for some large biotech firms. CROs are often used because their ability to reach out for regionalized patient populations and ability to efficiently manage international clinical studies. In any cases, however, the conventional role of CRO is to merely act as the “extra capacity” to facilitate R&D projects of the sponsors. By saving time, capital, labor, and/or space using specialized skills and/or research facilities of CRO, sponsors can focus more on the “core skills” and the growing regulatory demand that are relevant to their products.
Compared to other types of biotech firms, CROs have much better success rate as business entities either as small privately owned companies or as large international corporations. One of the important reasons is that CROs are less dependent on external funds such as VC than other biotech firms. CROs may survive on the service fees when external funds are not available for expansion. However, CROs usually do not request for the right of the potential intellectual property (IP) that may be generated from the services it provides. The matter of fact is that, most often, the part of the R&D project that CROs are involved are unlikely to generate any IP. The fact that the CRO is unable to claim for the right of the IP that may be generated through their own activities is probably the most obvious “downside” of the CRO business, especially when IP rights after Bay-dole act are usually claimed by research organizations even if the research might had been sponsored by the other parties. Therefore, CROs may be characterized as “low risk, low return” type of business and may be less attractive to investors who are looking for high return opportunities.
The global financial crisis may have created an opportunity for CROs to break the “low risk, low return” formula and enter the arena to compete for IP titles. As the financial support for start-up and early stage biotech companies quickly reduce to the very minimum, the so-called “valley of death” would become more “deadly” . As the result, new IPs are unlikely be developed to relatively maturation stages where the large and well-funded firms can see promising products and to invest further R&D efforts. Soon will see a shortage of relatively mature biotech drug candidates to supplement the inheritably insufficient in-house R&D of the established biotech firms. Having the infrastructures and funding in place for research and development, the profiting CRO may invest in early stage biotechnologies and seek for higher returns. CROs may not necessary become the replacement of small biotech firms, but an extra funding mechanisms for the desperate industry that is not on the “bail-out” list of the government.
Initiatives should be launched to invite CROs to invest in early biotechnologies. It is a risky idea for CROs to invest in early stage biotechnology. But the potential benefit of this initiative is not for CROs alone if “we” are seriously considering the challenges caused by lack of funds for early and small biotech firms. Here, I define “we” as the local government and established biotech firms because the pressure won’t be on the small and vanishing biotech firms’ shoulders alone if “we” are not going to do anything about the development pipelines that will soon be more broken in the “valley of death”.
Then the question comes to how to invite CRO to the IP hunting arena. The Maryland technology development corporation (TEDCO) had provided a good model by collaborating with Johnson & Johnson (J&J) to establish a co-managed funding agreement in 2005. This funding awards seed biotech companies with funds to develop technologies that are potentially of interest for J&J. TEDCO matches the J&J funds. J&J has opportunity for equity investment as the technologies mature (reference 5).
To invite CROs to joint collaborative research agreement, funding should be established by pooling money from government and sponsor companies to fund part (eg. 2/3) of a specific R&D project. CROs will identify and license the IP of interest and provide the rest (eg.1/3) of projected cost. Collaborations of the like will significantly increase the pipeline portfolio of early stage technologies for the sponsor with reduced risks without significant increase in R&D costs. CROs will obtain opportunities for generating IP assets without exhausting the profits earned from the contract works.
Local government, especially the state government, should take the initiative to invite CROs invest in early biotechnologies, not just to benefit CROs, but to stimulate the biotech industry and the economy of the state. Historical data showed that states that paid more attention to the biotech R&D received more returns from the industry. When the relative efforts in biotech R&D by states (national ranking of state expenditure in 2006) were plotted against the relative benefit received by states (national raking in patent number, VC investment, and NIH funding received), a clear positive correlation was seen between the state’s effort in R&D and the benefit the state received in a long run (Fig 2). For instance, California invested $6.5 billions (13.6% of total U.S. investment) in biotech R&D, ranking number one in the U.S. in 2006, indicating the involvement and focus of the state government. At the mean while, California received 24,293 patents between 2002-07 (nearly 20% of the national total), $20.7 Billion CV investment between 2002-07 (40.5% of national total), and $3.2 billion of NIH funding in 2007 (15% of national total), all ranked in number one on the national list.
State involvement and policy in biotech development play no doubt the most critical roles nurturing the growth of local biotech industry, including the CRO industry. Investing $1.7 billion in biotech R&D, North Carolina ranked in number eight on the national list in 2006, and now hosts two of the world-top-10 public-traded CRO companies, Quintiles transnational corporation and Pharmaceutical product development, making $3.57 billion of revenue in 2008. The state attracted capital investment from several internationally operating pharma/biotech companies. Recently, Novartis announced to build a $267.5 million vaccine manufacturing and Merk announced a $100 million expansion on its vaccine facility in the state. Constella Group, a private CRO company in Durham North Carolina grow from a statistical consulting service to a $200 million revenue-making bioinformatics firm in 2007 by assisting life-science clients.
In conclusion, encouraged and appropriately guided by the local government, CROs may deliver great value to the local economy. It’s important to recognize that the value of a CRO does not only exist in the revenue it generating, but also exists in its readiness to help the biotech industry overcome the current financial challenges.
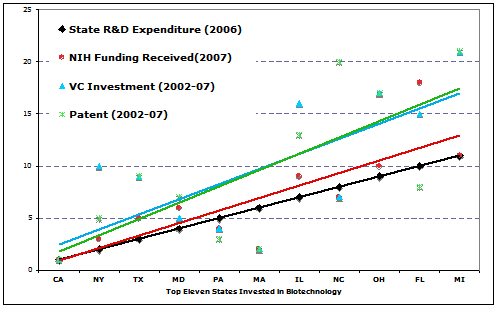
Fig 2. The correlation between the state R&D expenditure and the returns (number of patents, VC investment, and NIH funding to the state). The colored trend lines are regression lines corresponding to the data points of the same color. The Y-axis is the national ranking of the state with smaller numbers indicating the top states. (data source: reference 6)
1. US Pharmaceutical Industry Report, 2008-2009: www.reportlinker.com/p0118600/US-Pharmaceutical-Industry-Report-
2. Business insights: www.globalbusinessinsights.com/content/rbcr0001m.pdf
3. Global market review: http://knol.google.com/k/krishan-maggon/global-biotechnology-market-review/3fy5eowy8suq3/16#
4. IMS health: http://www.imshealth.com/portal/site/imshealth/
5. Maryland TEDCO: http://www.marylandtedco.org/tedcoprograms/ fundingopportunities.cfm
6. Biotechnology Industry Organization: http://www.bio.org/speeches/pubs/er/statistics.asp