This paper is a final project from the NIH Foundation for Advanced Education in the Sciences TECH 366, a course in Biotechnology Management
Therapies for Orphan Diseases: Public-Private Partnership in Drug Development
Research and development (R&D) of new drugs and therapies is both technologically and financially challenging. As return on investment (ROI) in R&D is largely driven by market demands and incentives, rare and neglected diseases (collectively known as orphan diseases), by their very nature, have limited commercial attraction to the pharmaceutical and biotechnology (Pharma/Biotech) industry (Nwaka and Ridley, 2003).
What are rare diseases?
In the US, a disease is classified as a rare disease when the prevalence of a particular disease in the US is less than 200,000 individuals. According to the National Institutes of Health (NIH) Office of Rare Disease Research (ORDR), there are ~6,800 rare diseases. About 25 million Americans are afflicted with these rare diseases. To date, only ~300 of the rare diseases have effective pharmacologic treatments. No effective treatments have been developed for ~6,500 of the rare diseases and this translates to ~20 million Americans (i.e., roughly 6-7% of the US population).
What are neglected diseases?
Diseases that are especially endemic to low-income populations in developing regions of Africa, Asia, and the Americas are called neglected diseases. According to the World Health Organization (WHO), worldwide, these groups of tropical diseases affect about 1 billion people (i.e., roughly15% of the world’s population). Even though there have been significant and rapid advances in deducing the pathogenesis of these neglected diseases, the drug ‘pipeline’ for these neglected diseases is almost dry (O’Connell, 2007). Therefore, there is a tremendous need to develop and deliver effective therapies for these neglected diseases.
Developing drugs for orphan diseases
For the millions of individuals afflicted with orphan diseases, new drugs are not likely to reach them in their lifetime. This is primarily because: (i) about 80-90% of the proposed drug candidates never even make it through clinical trials (high failure rate), (ii) the time required to bring a drug to the market is ~15 years (long development cycle), and (iii) on an average, the cost of bringing a new drug to market is about $850 million (high investment).
Historically, there had been limited interest from the Pharma/Biotech industry in developing novel therapies for orphan diseases. This has largely been due to the following factors: (i) low prevalence, and/or (ii) low commercial potential (i.e., low ROI). Consequently, there is a huge need for developing treatment for orphan diseases.
In order to encourage Pharma/Biotech companies to develop drugs for diseases that have a small market, the US Government, in 1983, passed the Orphan Drug Act (ODA; Public Law No. 97–414). This law provides the Pharma/Biotech companies attractive incentives to develop new treatments for orphan diseases including, tax breaks, market exclusivity for 7 years, tax incentives for clinical trials, etc. To further increase public investment in diagnostic tools and treatments for patients with orphan diseases, the US Government, in 2002, passed the Rare Diseases Act and the Rare Diseases Orphan Product Development Act (Public Law No. 107-280). Similar legislations to promote drug development for orphan diseases have been passed by the European Union (EU; 1999), Australia (1998), Singapore and Japan (1993; Braun et al., 2010).
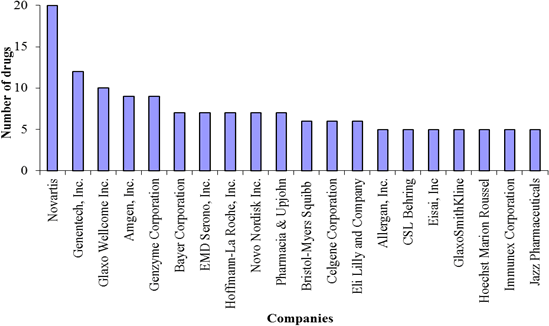
These favorable legislations have made the orphan products market lucrative and have spurred the Pharma/Biotech industry to develop drugs for orphan diseases (Coté et al., 2010). Since passing the ODA in 1983 and until the end of March 2011, the Food and Drug Administration (FDA) has granted 2,348 orphan designations. Of these, 369 have been approved for orphan indications. A majority of these drugs have been developed by big Pharma/Biotech – companies like Novartis, Genentech, Genzyme, etc. are leading the way (Figure 1).
Figure 1: Companies with 5 or more approved orphan drugs
Source: Data from FDA: http://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm. Note: this does not reflect the recent mergers and acquisitions.
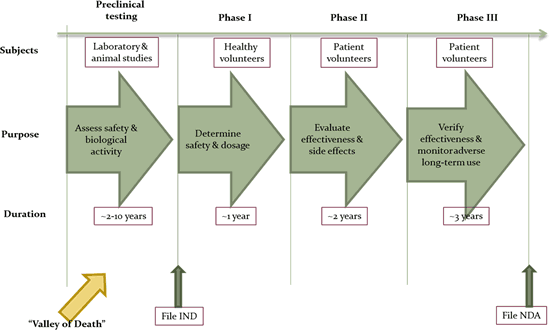
Although these favorable legislations have raised awareness and increased the effort to study orphan diseases, effective treatments for the vast majority of these diseases have not yet been developed. One of the major reasons is the high failure rate in the preclinical stages of drug development. A vast majority of the target drug candidates never make it past the ‘Valley of Death’ (Figure 2). This alone poses sufficient risk for the Pharma/Biotech industry to pursue development of orphan drugs. A good solution to this conundrum is ‘public-private partnerships’ (PPP) – collaborative partnerships that can help reduce the risks and costs for both the public and the private sector entities.
Figure 2: The long pathway to drug development
Note: IND – investigational new drug; NDA – new drug application.
What is a public-private partnership?
A public-private partnership (PPP) is a formal or an informal contractual arrangement between public sector(s) and private sector(s) that is created in order to achieve a public health objective or to produce a health-related product or service (Farrugia et al., 2008). In this case, to develop therapies for orphan diseases, public funds are utilized to engage both the public and the private sector researchers. These partners share certain risks and may exchange intellectual property, financial, in-kind, and/or human resources in any mutually agreed upon proportion (Lazdins-Helds, 2008). The ultimate goal of creating PPPs is to ensure that the products reach the patients who need them.
TRND: bridging the gap between drug discovery and drug development for orphan diseases
One of the initiatives that helps to leverage public investment and resources towards developing therapies for orphan diseases is the US Government supported initiative through the National Institutes of Health (NIH), called the Therapeutics for Rare and Neglected Diseases (TRND) program. This initiative is meant to jumpstart the development of drugs that may otherwise be ignored by the Pharma/Biotech industry.
The congressionally allocated budget for the TRND program was $24 million in both 2009 and 2010, and the President’s recommended budget for 2011is $50 million. The governance and oversight is provided by the NIH ORDR, while the laboratory operations are administered by the National Human Genome Research Institute (NHGRI). Depending on the nature of the particular project, other NIH centers also participate in the initiative. The TRND program is scheduled to become a part of the National Center for Advancing Translational Sciences (NCATS) in the future.
The main aim of the TRND program is to help move candidate drugs that have the potential to treat orphan diseases forward in the drug development pipeline until they meet FDA’s requirements for an investigational new drug (IND) application. TRND is a drug discovery and development program that provides support for specific aspects of preclinical research and drug development. Once a candidate drug is sufficiently ‘de-risked’, TRND hands it over to the external partners (e.g. pharmaceutical companies, disease-oriented foundations, etc.) to take the drug further in the drug development process.
Unlike the previous public initiatives (incentives, grants, etc.) for development of drugs for orphan diseases, TRND is a unique PPP program because it seeks to provide the drug development expertise, the technological know-how, as well as resources and services required for developing orphan drugs. Some of the expertise/services/resources provided includes medicinal chemistry optimization (production of dosage forms, stability testing), evaluation of functional activity, potency, pharmacokinetics (PK), pharmacodynamics (PD), efficacy, development of pharmacology assays and biomarkers, planning of clinical trials, IND filing advice, etc.
The TRND program is not a grant application, but rather an application to collaborate with experts and gain access to resources related to drug development for orphan diseases. This program stimulates research collaborations/partnerships between the industry and the academic scientists working on orphan diseases.
An example of PPP
A good example of PPP is the collaboration of TRND with AesRx, a company that is developing therapy for an orphan indication, sickle-cell disease (SCD; or sickle-cell anemia (SCA)). SCD is an autosomal recessive genetic blood disorder that is characterized by abnormal, rigid, sickle shaped red blood cells that block blood flow in the blood vessels and leads to various life threatening complications. Blood and marrow transplants offer a cure for small number of patients; however, there is no widely available cure for SCD. Majority of the therapy for SCD is targeted towards pain prophylaxis to help manage the symptoms and complications of the disease. Even with the best supportive care, the average lifespan is only about 40 years. Even though a number of potential drugs have been studied, the only drug approved for the treatment of SCD is the anti-cancer agent hydroxyurea which is associated with a number of side effects. Hence, there is a significant unmet medical need for developing a novel treatment for SCD.
Aes-103 is a potential therapy for sickle cell disease
AesRx is a biopharmaceutical company that is focused on the development of two novel drugs that targets two different orphan diseases. Of these two drugs, the lead program is a drug called Aes-103 (5-hydroxymethyl-2-furfuraldehyde (5HMF)) which has the potential to treat SCD. The prevalence of SCD is less than 200,000 in the US and it is highly prevalent in Asian and African countries (Table 1). Because of the low US prevalence of SCD, Aes-103 received the orphan designation from the FDA in 2006, which in turn qualifies it for an accelerated FDA review.
Table 1: Prevalence of SCD in the US and rest of the world.
| DEMOGRAPHICS | PREVALENCE | PROPOSED MARKET |
| USA | 75,000 | Primary |
| EU | 40,000 | Primary |
| India | > 1,000,000 | Secondary |
| Middle-East | 100,000 | Secondary |
| Africa | >12,000,000 | Tertiary |
| Total | >13,000,000 |
5HMF increases the affinity of sickled hemoglobin for oxygen (Abdulmalik et al., 2005). 5HMF and other related hydroxymethylfurfurals (HMFs) have been identified in a wide variety of heat-processed foods. Consequently, a large volume of safety data (estimated to be worth about $3.5 million) is available from a number of toxicological studies have already been performed by the scientific community. Hence, Aes-103 has been sufficiently ‘de-risked’ as human and animal safety data was already available. However, for IND submission, other additional pharmacological and toxicological preclinical data are required.
Aes-103 has also benefitted from 2 NIH grants: (i) a Small Business Innovation Research (SBIR) grant to further the preclinical development process, and (ii) Rapid Access to Invention Development (RAID) grant for cGMP manufacture. In spite of these grants, Aes-103 was pushed to the brink in the ‘Valley of Death’ (Usdin, 2010). AesRx was unable to raise the several million dollars required to complete the preclinical studies required to file an IND. This is because the current model of financial investment in drug discovery and development is changing. In these economically trying times, venture capitalists (VCs) and big pharmaceutical companies are not willing to make an investment until some human proof-of-concept data is available. These investors are moving away from investing in preclinical studies and are more willing to invest later in the drug development cycle – late Phase II or III, i.e., well past the ‘Valley of Death’. As a result, there is a funding gap at the preclinical stage and consequently, a number of compounds are left to languish in the ‘Valley of Death’.
TRND-AesRx partnership to develop Aes-103
Aes-103 was teetering at the rim of the ‘Valley of Death’ when TRND stepped in to rescue it. Aes-103 is one of the first molecules to enter the TRND program. For TRND, Aes-103 was attractive because: (i) it fits the goals, i.e., huge need for therapy in an unaddressed market, (ii) the proposed mechanism of action had already been validated, (iii) it is orally bioavailable, (iv) the compound was sufficiently ‘de-risked’ as human and animal safety data was already available, and (v) patents had already been issued. These factors led to a reduction in the lead optimization that is required for further development of Aes-103. However, IND application requires a number of other toxicological and preclinical studies as well.
Apart from saving Aes-103 from the ‘Valley of Death’, TRND fits the other needs of AesRx. As a small, ‘virtual’ company, AesRx outsources most of its R&D. Hence, a partnership with TRND, with all its drug development resources helps to move the preclinical and a part of the clinical studies forward.
Upon finalizing the TRND-AesRx collaboration, a project team was formed between the two entities to discuss and develop a project plan. This project plan included details such as development plan, timeline, milestones and deliverables, and Go/No-Go decision points. If these criteria are not met, the project can be terminated. As far as the intellectual property (IP) is concerned, the IP owned by AesRx (the ‘institution’s IP’) will be used in the project. Any new IP created from the TRND-AesRx partnership will be determined according to the patent laws.
AesRx expects to file for an IND for Aes-103 in the fourth quarter of 2011, provided that all of the preclinical hurdles can be crossed. The AesRx-NIH collaboration will continue even after the IND has been approved. AesRx will work with National Heart, Lung and Blood Institute (NHLBI) for Phase I and IIa clinical trials, expected to begin sometime during late 2011 or early 2012. This clinical trial is expected to provide mechanistic proof-of-principle, identify biomarkers of clinical activity, determine dosage, etc. Aes-103 is expected to meet the objectives of TRND in 2013-2014. Once the proof-of-concept is established, AesRx anticipates on working with external partners (e.g. pharmaceutical companies) to move the compound forward. A pharmaceutical partner has not been identified yet. However, as Africa is expected to be AesRx’s largest market by size, albeit, a tertiary market, drug distribution expertise in Africa will be desirable. In terms of financial measures, AesRx expects to make its ROI through its primary markets – US and EU. In addition to the primary market, the secondary markets are the Middle East and India, while the tertiary market is sub-Saharan Africa. If the drug is approved, Aes-103 will enjoy 7 years of exclusivity. The annual market potential is expected to be over $1 billion (Source: www.onemedplace.com/database/list/cid/14087/).
PPPs do not solve all the problems
For AesRx, artnership with TRND is not the panacea for all problems. While, TRND provides resources and services for orphan drug development, it does not provide any direct monetary support. Even though AesRx is a small, ‘virtual’ company, it still needs funds to cover its overhead costs. Consequently, the company has to look beyond the TRND program to support itself monetarily. One such source of financial support has been through the Accelerator Program loan from the Massachusetts Life Science Center – AesRx was recently awarded a loan of $750,000.
Summary
In summary, there is a huge need for developing drugs for orphan diseases. Public-private partnerships (PPP) can help develop drugs for these diseases. Such partnerships reduce risks and costs for both public and private sector partners. These PPPs encourage the development of new safe and effective medical products for orphan diseases and in the process help to achieve a critical public health objective. However, PPPs are not an end-all solution for the woes associated with drug development. The current model of drug development requires significant further change to encourage and sustain the ‘pipeline’ over a long run, while still delivering safe and effective drugs to meet the growing challenges related to human health.
Acknowledgements
I am thankful to Christopher P. Austin, M.D., Director, NIH Center for Translational Therapeutics and Stephen R. Seiler, Founder and Chief Executive Officer, AesRx for providing some of the details pertaining to TRND-AesRx partnership.
References
- Abdulmalik, O., Safo, M.K., Chen, Q., Yang, J., Brugnara, C., Ohene-Frempong, K., Abraham, D.J., and Asakura, T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. British Journal of Haematology, 128 (4): 552–61 (2005).
- Braun, M.M., Farag-El-Massah, S., Xu, K., and Coté, T.R. Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nature Reviews Drug Discovery, 9(7): 519-22 (2010).
- Coté, T.R., Xu, K., and Pariser, A.R. Accelerating orphan drug development. Nature Reviews Drug Discovery, 9(12):901-2 (2010).
- Farrugia, C., Reynolds, T., Orr, R. J. Public-Private Partnership Agencies: A Global Perspective. Collaboratory for Research on Global Projects. Working Paper #39 (2008).
- Lazdins-Helds, J.K. Drug development through public private partnerships (PPP). Presented at the Symposium on Public Sector IP Management in the Life Sciences on December 15, 2008.
- Nwaka, S., and Ridley, R. ‘Virtual’ drug discovery and development for neglected diseases through public-private partnerships. Nature Reviews Drug Discovery, 2: 919−928 (2003):
- O’Connell, D. Neglected Diseases. Nature, 449: 157 (2007).
- Orphan Drug Act, H.R. 5238, Public Law No. 97–414, 97th Congress (1983).
- Rare Diseases Act and Diseases Orphan Product Development Act. Public Law No. 107-280, 116 Stat 1988 (2002).
- Usdin, S. Translation under one roof. Science–Business eXchange (2010).
Web links
- Food and Drug Administration – orphan drug designations and approvals – http://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm
- National Heart, Lung and Blood Institute (NHLBI) – Sickle cell anemia (SCA): http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhatIs.html
- National Institutes of Health (NIH) – Office of Rare Disease Research (ORDR): http://rarediseases.info.nih.gov
- Therapeutics for Rare and Neglected Diseases (TRND): http://trnd.nih.gov/
- World Health Organization (WHO) – neglected tropical diseases: http://www.who.int/neglected_diseases/en/