Much has been written on the controversial price increase of Daraprim.
Daraprim treats malaria and another parasitic infection, toxoplasmosis. It was acquired by Turing Pharma from Impax Laboratories and shortly after the price was raised from $13.50 a tablet to $750 a tablet.
The led to a quick outcry, followed by a) a defense by Turing of their drug pricing and b) a promise to decrease the price.
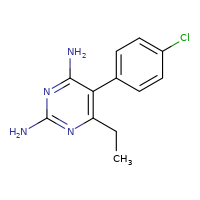
Almost immediately, I was asked what the patent situation was for Daraprim, and if it was possible for generic manufacturers to produce pyrimethamine, the active ingredient in Daraprim.
Consulting the DrugPatentWatch database shows that Daraprim was approved decades ago (1953, in fact) and has no patent protection. Politifact gets it wrong and says that the patents expired in 1953, but in fact they were granted in the range of 1950-1954, likely expiring in the late 60s and early 70s.
This begs the question: Why aren’t generic manufacturers producing and selling generic Daraprim? The answer likely lies in sales volume. According to IMS data cited by the New York Times, last years’ sales for Daraprim were $9.9 million. Given that top-selling drugs may have sales volumes in the billions of dollars, Daraprim is simply too small a target for generic manufacturers.