New patent expiration for Titan Pharms drug PROBUPHINE
Annual Drug Patent Expirations for PROBUPHINE Probuphine is a drug marketed by Titan Pharms and is included in one NDA. There is one patent protecting this drug. This drug has…
Source
Annual Drug Patent Expirations for PROBUPHINE Probuphine is a drug marketed by Titan Pharms and is included in one NDA. There is one patent protecting this drug. This drug has…
Source
[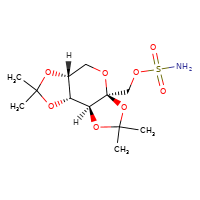](//www.DrugPatentWatch.com/p/preview/generic-api/topiramate?utm_medium=dpw_wp_blog&utm_campaign=dpw_wp_blog&utm_source=dpw_wp_blog) Topiramate is the generic in…
Get new actionable insights and updates from BiotechBlog