December 29, 2021 Comments Off on New tentative approval for Hospira Inc drug bortezomib Posted in: Tentative Approval [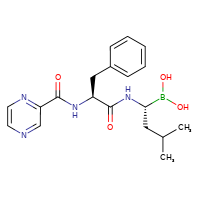](//www.DrugPatentWatch.com/p/preview/generic-api/bortezomib?utm_medium=dpw_wp_blog&utm_campaign=dpw_wp_blog&utm_source=dpw_wp_blog) Bortezomib is the generic in…
December 28, 2021 Comments Off on New patent expiration for Mylan Speciality drug DUONEB Posted in: Uncategorized
Annual Drug Patent Expirations for DUONEB Duoneb is a drug marketed by Mylan Speciality Lp and is included in one NDA. There is one patent protecting this drug. This drug…
The post New patent expiration for Mylan Speciality drug DUONEB appeared first o…
