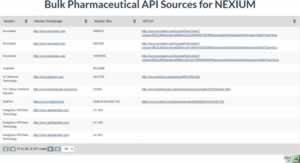
New features from DrugPatentWatch – Bulk API sources and clinical trials
After months of development and feedback from customers, DrugPatentWatch is proud to announce the availability of two new datasets: Bulk […]
New features from DrugPatentWatch – Bulk API sources and clinical trials Read Post »